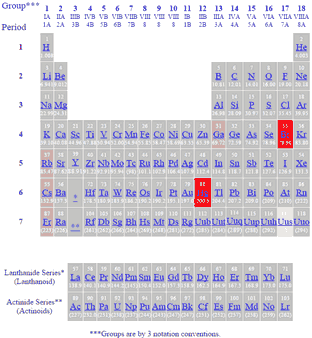
In the periodic table above black squares indicate elements which are solids at room temperature about 22ºc those in blue squares are liquids at room temperature and those in red squares are gases at room temperature.
Identify the elements that are liquid at room temperature.
Point at or click an element in the periodic table for more information.
Although elements caesium cs rubidium rb francium fr and gallium ga become liquid at or just above room temperature.
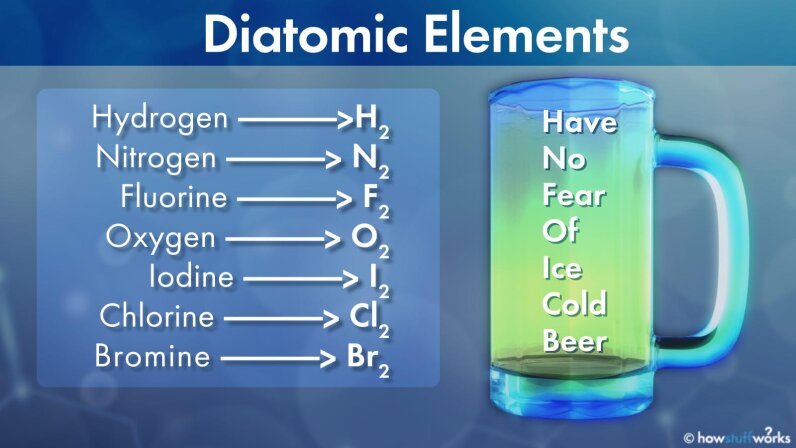
It is a form of homonuclear diatomic molecule.
For science it s usually considered to be either 20 c or 25 c.
Liquids stp and liquids around room temperature the only liquid elements at standard temperature and pressure are bromine br and mercury hg.
Photographs and descriptions of many samples from the collection gas at room temperature in the periodic table.
Gas at room temperature 13 these elements are gasses at room temperature and pressure.
There are 7 diatomic elements but only 5 diatomic elements at standard temperature and pressure the diatomic elements are hydrogen nitrogen oxygen fluorine chlorine bromine and iodine.
At this temperature and ordinary pressure only two elements are liquids.
A diatomic element is a molecule of an element consisting of two atoms.
Elements that are liquid at 25 c.
Radon helium xenon neon krypton and argon are eight noble gases.
Each of the 13 elements has their own unique physical and chemical properties.
Click any element below to see all the samples of that element.
Most of the metals are solids under ordinary conditions i e 25ºc 1 atmosphere of pressure etc with the exception of mercury hg element 80 which solidifies.